Glass shatters the myths.
 Glass: past elegant, future thin
Glass: past elegant, future thin- Glass artefacts make life pleasanter as well as easier, and now they promise to form a vital part of networks that will carry speedy messages across a data-hungry world. Its chemistry has changed only in detail: it remains a simple inorganic substance with a locked-in liquid structure.
Glass
John Emsley
Published: 8 December 1983
From the time of its discovery, four-and-a-half-thousand years ago in the Old Kingdom of Ancient Egypt, until the end of the 19th century, the beauty and versatility of glass more than met the needs of artist and builder alike. In the 20th century, research into its basic chemistry have engendered quite new kinds of glass, and new industries: toughened glass, glass fibre, glass ceramics and optical fibres – this last alone could transform society within a decade.
Yet glass, basically, is remarkably simple: a mixture of silicon dioxide and metal oxides. What gives glass such remarkable properties is its atomic structure. Neither a liquid nor a true, crystalline solid, it is a compromise between the two: a supercooled liquid. At first glance glasses appear to be solid enough, but if we inspect their internal structure with X-rays we do not find the regular and orderly packing of atoms found in other solids. The structure is more akin to the random arrangement of a liquid, which is what it is, but a liquid cooled below its expected freezing point. Such a liquid is very viscous, glass itself being so viscous that it hardly deforms under the pull of gravity. In this respect it behaves deceptively like a solid.
Chemists explain the difference between a crystalline solid and a glass by saying that the former has long-range order whereas the latter has not. Long-range order means that the same stacking of atoms is repeated in a pattern at regular intervals throughout the whole crystal. X-rays passing through such a crystal reveal this underlying arrangement by the systematic way in which they are deflected. X-rays going through a glass or a liquid are deflected in all directions showing the disordered array of atoms. Figure 1 shows this difference in two dimensions between a crystal and a glass.
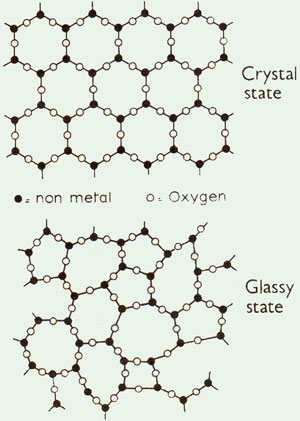 Figure 1
Figure 1- The characteristic order of a crystal such as quartz (above). After melting disorder is lacked in when the substance solidifies (below)
To solidify, a liquid must be composed of freely moving molecules or atoms so that these can arrange themselves neatly into a crystal lattice. In glass all the atoms are bonded to one another by attractive forces. As a result, independent motion is lost and rearrangement into a symmetric crystal array at room temperature is too slow to measure. Pieces of ancient Egyptian glass have still not crystallised after 4000 years.
Most glass is made from sand, limestone and soda ash. Sand is almost pure quartz, which is silicon dioxide, SiO2. In this material each silicon atom is covalently bonded to four oxygen atoms in a perfectly regular crystal (Figure 2). Quartz melts at 1710°C and once molten this order is lost. The ancients could not produce such temperatures, but they could reach 1000°C with charcoal furnaces, and it was in these that they must have discovered that sand, when mixed with other minerals, could be made to melt and form a glass. The other minerals are limestone, which is calcium carbonate, (CaCO3), and soda ash, which is sodium carbonate Na2CO3). Egypt is one of the few places where sodium carbonate occurs naturally.
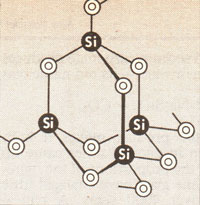 Figure 2
Figure 2- Three-dimensional structure of quartz (SiO2).
Heating together just sand and sodium carbonate produces sodium silicate, which is soluble in water–its old name was water-glass:
Na2CO3 + Na2SiO → Na2SiO3 + CO2
The presence of calcium in the product makes it both insoluble and harder. Craftsmen in ancient times came up with a good recipe for glass, and the proportions of the ingredients used to make this soda-lime glass have changed little over 4000 years. A typical glass contains (by weight) 70 per cent SiO2, 15 per cent sodium oxide (Na2O) and 10 per cent calcium oxide (CaO), with 5 per cent of other oxides. In fact sodium and calcium are added as carbonates and then lose carbon dioxide (CO2) to form sodium oxide, and calcium oxide in the glass. This glass has a softening temperature of around 650°C, making it easy to work and blow.
Metal oxides have to be present because they alter the chemical bonding in the glass, which in turn lowers the melting temperature and the viscosity. A structure in which every atom is covalently bonded to its neighbours in three dimensions is very strong as shown by quartz, which can survive millions of years of weathering. Break some of these bonds and the structure is weakened: Figure 3 shows how sodium oxide brings this about. For each pair of sodium ions, Na+, in the glass, or for each calcium ion, Ca+, one Si-O-Si covalent bond must break. As a result the melting point is lower; in soda glass it is about 1000°C lower than that of pure quartz.
-
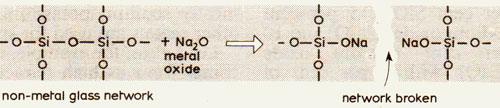
- Figure 3 How sodium oxide breaks bonds, thus weakening the structure of the glass, and lowering the melting point.
We can, therefore, talk of two distinct constituents of glass: the non-metal oxide, called the glass-former or network former, and the metal oxide, called the network modifier. Silicon is not the only glass-former. Boron, aluminium and phosphorus are also suitable. The network modifiers are mostly sodium, potassium, calcium and magnesium, but other metals are used for special effects.
Lead glass, for example, is known for its brilliance, which comes from its high refractive index. This was patented in 1674 by George Ravenscroft, who had been commissioned by the Worshipful Company of Glass Sellers of London to experiment on native minerals as a basis for a British glass industry. In those days Venice had a virtual monopoly of European glass. Ravenscroft came up with flint glass (flint is a type of quartz) to which he added lead oxide to improve its transparency. It quickly caught on and its beauty, enhanced by grinding into cut glass, led to its widespread use in ornaments and tableware.
Because it is basically a liquid, glass is transparent. As there are no internal boundaries in a liquid there is no internal refraction or reflection of a beam of light within it. A ray of light can pass straight through glass, although it is refracted slightly when it crosses the surface of the glass. Luckily, glasses formed from oxides are transparent to that part of the electromagnetic spectrum which includes visible light: hence their popularity.
Dramatic changes in the light which a glass will absorb or let through can be made by putting small amounts of transition metals in the molten glass. Cobalt gives a blue glass; manganese, purple; chromium, green; copper, blue-green; and so on. The earliest glass artefacts were coloured, though the art of colouring glass reached its peak in the Middle Ages.
Glass is naturally yellow-green because of the trace of iron present in sand, which produces Fe2+ ions in the glass; and the glass has to be decoloured by adding manganese dioxide (MnO2) which this oxidises the Fe2+ to the much paler Fe3+. Later it was discovered that small amounts of selenium will also decolour glass and only 30g (1 ounce) per tonne of glass will suffice. In higher concentrations the selenium tints the glass red.
As many of us know to our cost, the soda-lime glass used in windows is prone to shattering, exposing dangerously sharp edges. Sudden changes in pressure or temperature will fracture it, and overcoming these drawbacks has motivated much research into glass. The first major step forward came when researchers added boron as part of the glass-former. Pyrex glass was the result.
This was first made in 1912 by Eugene Sullivan and William Taylor at the Corning Glass Company in the US. A small amount of boron oxide, B2O3 – 10 to 15 per cent will do – makes glass more resistant to shock by blows or heat. Borosilicate glass is also more resistant to chemical attack, and is now the universal material for glassware for chemical and biochemical research. The only common reagent which corrodes it is hydrofluoric acid, HF, which can convert boron to BF4- and silicon to SiF62-. Both are soluble in water.
Below boron in the periodic table of the elements is aluminium. A few per cent of this in glass raises the melting point and increases still further its resistance to chemical attack. This is particularly important at the surface if the surface area is very large, as in glass fibre.
Fibres of glass are produced naturally by high winds blowing across the surface of molten lava from volcanoes such as Mount Etna in Sicily. The ancient world spoke of this material as Goddesses' Hair.
Later, the Venetians used glass filaments to decorate their products. These soda glass filaments are too easily broken for use as glass fibre, but aluminosilicate glass fibre can be used to make insulation, to reinforce plastics and cements and can even be woven into fabrics.
Glass can be toughened by rapid surface cooling, dipping into molten potassium chloride (KCl) or spraying with a titanium compound. This last technique allows lighter bottles to be made and increases the operating life of recycled bottles such as those for milk or soft drinks. Even so, the use of glass for bottles is rapidly declining: clear, unbreakable polyester plastics containers are shouldering them out.
Before the discovery of fibre optics the most important British contribution to glass technology was the invention of float glass at Pilkington, St Helens, Lancashire. The brain-child of Alastair Pilkington, this process saved them up to 30 per cent of sheet glass that had to be ground away to produce plate glass. This year the firm signed an agreement with the People's Republic of China to set up a plant at Shanghai, and there are now 90 such plants throughout the world. The glass is essentially crude soda-lime glass.
At the other end of the scale the glass for optical fibres must be ultra-pure, and this is the reason for its high cost: £l per metre. This glass is made from silicon tetrachloride (SiCl4), a liquid that boils at 58°C and one which can be purified by distillation. The first step is to convert SiCl4, to silicon dioxide by heating with oxygen gas at 1200°C. This chemical reaction is conducted inside a quartz glass tube so that the silicon dioxide is deposited as a layer on the inside walls. When this layer is about l mm thick a second component is introduced along with the SiCl4 so as to form an inner deposit that has a high refractive index. This layer eventually becomes the glass along which the light waves travel. The other component is either germanium tetrachloride, GeCl4, or titanium tetrachloride, TiCl4. These oxidise to GeO2 and TiO2.
When the second layer is thick enough the whole tube is heated until it collapses into a solid rod of glass, this is heated to 2200‐C at one end and a thread of molten glass drawn from it. This is the optical fibre with a diameter as fine as hair (150 x l0-6 m). To protect the fibre from chemical and mechanical damage it is coated with two layers of polymer such as an inner silicone layer and an outer nylon layer.
Optical fibre is the answer that inorganic chemistry has provided to the challenge of transmitting information over long distances. Using light waves passing along glass, as an alternative to electrons (electricity) flowing down a metal wire, optical fibre should eventually offer both lower capital costs and even lower running costs. Bending light round corners presents no difficulty to glass fibre provided the conditions are right for total internal reflection-that is, the light beam must strike the internal surface of the glass at less than a certain angle known as the critical angle. Above this angle the beam of light can escape from the glass.
The first ultra-pure glass fibre suitable for optical fibre was produced by Dr Keith Beales and Dr George Newns at British Telecom's research laboratories at Martlesham. They developed the double crucible method in which the glass for the fibre's core is held molten in a crucible within another holding molten glass for the outer layer. The optical fibre is pulled from the two crucibles together in a continuous thread. This kind of fibre is called multimode optical fibre and has a wider core than monomode fibre. On the other hand multimode fibre can carry several beams of light at the same time whereas monomode, as its name implies, carries only one. Nevertheless, monomode is preferred for its clarity of signal and the greater distances over which this can be carried.
Three British companies currently produce optical fibres at a combined rate approaching 100 000 kilometres per year. These are: Standard Telecommunications at Harlow, Essex; GEC Optical Fibre which is headed by Dr Steven Cundy, a Cambridge physicist turned manager. The third, Optical Fibres of Shotton on Deeside in Wales, is one of the Welsh Development Agency's pride and joys. Standard Telecommunications, has won a contract for part of the transatlantic telephone cable.
Optical fibre is made as pure as possible to ensure there is minimum loss of light as it passes along it. A glass to prevent the passage of light can be made by incorporating silver chloride (AgCl), which darkens on exposure to sunlight–just as it does on a photographic plate. The silver ion Ag+ is converted to an atom of metallic silver by picking up an electron. In photosensitive glass the atom of silver again loses its newly acquired electron when the light fades, reverting to the colourless Ag+ ion. This is the glass used in certain types of sunglasses.
Photosensitive glass led indirectly to an even more important discovery–glass ceramics. S.D. Stookey, a research chemist at Corning Glass, was at first dismayed when he found he had accidentally overheated a sample of such glass in his furnace overnight. It had become permanently opaque, and apparently useless. However, it had also become almost unbreakable, either by mechanical shock or heat shock. Today we see glass ceramics in ovenware, pipes and electrical insulators. It also appears in such exotic places as rocket nose cones and space-shuttle tiles.
Glass will crystallise or devitrify if you heat it to about 600°C, just below its softening point, for several months. Crystallisation can be speeded up to a matter of hours if certain substances are present in the glass such as silver, titanium or phosphorus. These act as "seeds" from which crystals can start to grow. At 500–750°C the glass will start to reform into the perfect arrangement of a crystal.
These properties of the new material can vary enormously. A glass ceramic of composition 74 per cent SiO2, l6 per cent aluminium oxide (Al2O3) 6 per cent titanium oxide (TiO2), and 4 per cent lithium oxide (Li2O) doesn't even expand on heating. If zinc oxide is used in place of aluminium then the material has a very high resistance to fracture. A glass of percentage composition 60.2 SiO2, 28.5 Al2O3, 8.5 LiO2 and 2.8 magnesium oxide (MgO) expands on heating and softens at 720°C. Glass ceramic of exactly the same composition actually shrinks on heating, and its softening does not begin below l0O0°C. Generally, glass ceramics outperform glass in almost every respect but one, transparency.
And so research into glass continues to produce surprises. Cyril Drake, a researcher at Standard Telecommunications Laboratories has produced a glass without any silicon dioxide in the glass forming matrix. The glass has phosphorus oxide in its place and sodium and calcium as the network modifiers. The glass dissolves very slowly in water and is being tested as a medium for the controlled release of other substances. Copper is toxic to water snails which harbour the debilitating disease schistosomiasis (bilharzia), the disease that blights so many lives in Third World countries, and pellets of the glass, impregnated with copper, have been put into ponds and streams. Dr John Jewsbury of the School of Tropical Medicine at Liverpool University has used the glass in small-scale trials in Zambia with success. The phosphorous oxide glass seems ideal also for a slow-release fertiliser – and prospective manufacturers are currently mulling over the idea.
Chemists have even worked out how to make glass without heating the material to high temperatures. Lisa Klein of Rutgers University at New Brunswick in New Jersey has shown that the hydrolysis of silicon tetraethoxide, Si(OC2H5)4, produces SiO2. This first forms a gel and then a glass:
Si(OC2H5)4 + 2H2O → SiO2 + 4C2H5OH
The ethanol, C2H5OH, slowly evaporates from the semi-solid gel and eventually leaves behind a hard glass that is referred to as a monolithic dried gel. Heating speeds up the process. The product must be shaped at the gel stage and made larger than required because it shrinks during drying. There are two advantages to this method of glass forming-firstly, the glass can be made very pure because Si(OC2H5)4 can be distilled. Secondly, other materials can be introduced into this low-temperature glass that would decompose in the ordinary high-temperature processes. The product is expensive to make, however, so that only very special uses are likely to justify the cost of making monolithic dried gels.
Before the invention of glass ceramics the most stable glass that could resist mechanical and thermal shocks was the material discovered in 1930 by Martin Nordberg and Harrison Wood working at Corning Glass. This has the percentage composition 96 SiO2, 3 boron oxide (B2O3), 1 other oxide. It was called Vycor and was a cheaper alternative to fused quartz, for containers that had to withstand very high temperatures. Vycor can be plunged red hot into cold water and comes to no harm. It was made from borosilicate glass which was treated with hot nitric acid that leached out most of the metals in the glass to give a porous material. Heating this at 1200°C caused it to shrink by 14 per cent to give Vycor.
The porous glass from the acid etching has found an alternative use as support material for enzymes. These can be chained to the glass by covalent bonds by the reactions shown in Figure 4. The enzyme, immobilised on the glass, can still carry out its vital functions without being carried away in the process. The enzyme invertase can hydrolyse the sucrose carbohydrate of cane or beet sugar to the more useful carbohydrates glucose and fructose simply by letting a solution flow slowly over the supported enzyme. Moreover, the enzyme remains active for several months.
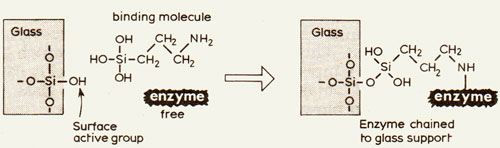
- Figure 4 An enzyme molecule is chemically "chained" to a glass support, so it can be used repeatedly.
Traditional products of the glass industry–such as bottles and jars–are being rapidly replaced by safer and lighter alternatives. The new highly specialised applications of glass will make it a more glamorous and expensive substance, but there will be far less of it around in our daily lives.