Hydrogen links up with other atoms in many ways, forming a wide variety of compounds, from methane to DNA. Latest research (in 1981) reveals the strong hydrogen bond – a previously unknown way for hydrogen to form compounds.

- Hydrogen is one of the most versatile elements. As the lightest of gases molecular hydrogen was used to fill airships; its bonds in ice produce a structure that floats on water.
The hidden strength of hydrogen
John Emsley
Published: 30 July 1981
Of all the chemical elements, hydrogen is the simplest in structure, and first in the diversity of its chemical behaviour. The element itself exists as the molecule H2 which is well known as the lightest of all gases. Although industry uses this gas on a large scale it is rarely encountered in everyday life except to fill balloons. However, in 25 years time this may be the gas which is piped into our homes to fuel boilers and cookers – once we have used up supplies of natural methane gas, CH4. Hydrogen burns to form water, and hence is cleaner than gases containing carbon.
Chemists find hydrogen particularly interesting because of the versatility of its chemical bonding, as apparent from the many different types of compound that it can form. The hydrogen atom consists of a nucleus containing a single proton with an electron in orbit around it. This orbit can accommodate at most two electrons, so hydrogen can form only one covalent bond – a bond in which a pair of electrons is shared by two atoms – to another element. This it does in most of its compounds. When bonded to carbon as in CH, and the many other organic compounds with C-H bonds, the hydrogen atom is firmly held by this covalent link to the carbon atom. On the other hand, hydrogen can form stronger bonds with oxygen yet still be mobile. Thus in water, H2O, the hydrogen atoms exchange between different oxygen atoms billions of times per second.
In some compounds, namely acids, the molecules are so averse to the hydrogen they contain that they will readily donate the hydrogen to other molecules. One such is hydrogen chloride, HCl, and textbooks often write this process as HCl → H+ + Cl-. But H+ is a "bare" proton, and as it has an overwhelming attraction to any electron pair in its vicinity it cannot exist apart from a molecule. Thus the H+ from an acid is drawn immediately to another molecule, especially to any atom within that molecule which has a pair of electrons in its outer shell, unattached to any other atom. Oxygen and nitrogen atoms are particularly attractive to H+ because of the lone-pair electrons they contain. Some acids, such as "magic acid", are so strong that they can donate their protons to almost any other molecule whether it has a "free" electron-pair or not. "Magic acid" is a mixture of fluorosulphuric acid, HSO3F, and antimony pentafluoride, SbF5. The acid is so strong that when sulphuric acid is dissolved in it, the sulphuric acid is forced to play the part of accepting a hydrogen and so becomes H3SO4+ !
The behaviour of acids is generally observed in water. The solvent (H2O) is the recipient of the proton, as for example in HCl + H2O → H3O+ + Cl-. H3O+ is called the oxonium ion and is commonly regarded as the key chemical species in acid solutions. But this is only part of the story. The oxonium ion combines with three further water molecules to form H9O4+. This complex ion is held together by hydrogen bonds. This type of chemical bonding is unique to hydrogen, hence the name. Recently chemists have become aware that hydrogen bonding is itself of two kinds – weak (or normal) hydrogen bonding and strong hydrogen bonding. The latter type has implications in several areas, especially in the chemistry of fluoride (F-); it also poses questions that current theories of chemical-bonding cannot answer.
The idea of weak hydrogen bonding was first proposed by an American PhD student, M. L. Huggins, in 1919. He used it to explain the peculiar properties of water, a liquid so common that we tend to take its unusual features for granted. For instance, the melting point and boiling point of water are far too high for such a light substance with a molecular weight of only 18 – H2S, H2Se and H2Te with molecular weights of 34, 81 and 130 respectively are all gases at room temperature. In addition, the density of water is higher than that of ice, whereas for all other compounds the solid phase is denser than the liquid phase.
Hydrogen bonds form between molecules because the hydrogen that is covalently bonded to one molecule becomes attracted to another molecule. It behaves in this manner when the covalent bond is polarised, which means that the electron-pair of the bond is attracted away from the hydrogen toward the other atom. This can occur when hydrogen is attached to nitrogen, oxygen, fluorine and a few other elements. In a polarised bond the hydrogen becomes exposed so that the positive charge of the proton nucleus can attract a region of negatively charged electrons, such as a lone-pair, on another molecule. This attraction is enough to pull the two molecules together; the weak link they form is called a hydrogen bond and between two water molecules this is depicted as H-O-H⋅⋅OH2, where the two dots indicate the hydrogen bond.
The strength of such hydrogen bonds is 10–30 kJ/mole (where a mole contains approximately 6 x l023 molecules), this being the energy required to separate the pairs of molecules again. It is only a small fraction of the energy required to break a covalent bond, which for the O-H bond of water is around 400 kJ/mole. Moreover, the distance of the hydrogen from the other larger atoms is different in the two types of bond. In O-H⋅⋅O, for example, the O-H covalent bond is about 100 picometres (1 pm=10-12 m) whereas the H⋅⋅0 bond is about twice as long.
Chemists have found the concept of hydrogen bonding useful in explaining many of the irregularities found in compounds with O-H and N-H bonds. For example, acetic acid, CH3CO2H, behaves as if it were (CH3CO2H)2, and we can see why when we realise that it is suited to forming such dimers (molecular pairs) because of its potential for hydrogen bonding (Figure 1).
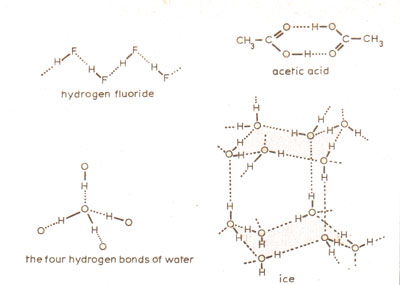 Figure 1
Figure 1- Weak (or normal) hydrogen bonds–the dots–hold together a variety of materials. Ice crystallises in a structure with large cavities which collapse when it melts
The technique of X-ray crystallography has enabled researchers to elucidate the structures of molecules, but it has provided only indirect proof of the existence of hydrogen bonds. This is because X-rays produce images of a substance when they are deflected by electrons, which are in rather short supply around the hydrogen in a hydrogen bond. Although it is possible these days to use sophisticated techniques to locate the hydrogen atoms with X-rays, to pin-point them accurately researchers must use beams of neutrons, which are deflected not by the electrons but by the nucleus itself.
The most important of all hydrogen bonds are those which hold together the double helix of DNA, deoxyribonucleic acid, the stuff of which genes are made. Francis Crick and James Watson first realised in 1953 that hydrogen bonds were responsible for interlinking the two strands of DNA, basing their speculations on the experimental X-ray work of Maurice Wilkins and Rosalind Franklin. (This discovery earned Nobel prizes in 1962 for the three men involved but not for Rosalind who died in 1958 aged 57 – a story told by James Watson himself in The Double Helix (1968) and slightly more objectively by Anne Sayre in Rosalind Franklin and DNA (1975).) The hydrogen bonding in DNA is between the two pairs of bases: adenine with thymine, and guanine with cytosine (Figure 2). These combinations are ideally suited to form hydrogen bonds with each other. As the strands of DNA form, each base seeks out its counterpart and ensures a perfect copy of the original double helix.
 Figure 2
Figure 2- Probably the most important (weak) hydrogen bonds of all are those that hold together the two strands of DNA – the stuff of which genes are made. The hydrogen bands link the pairs of bases – T to A, and C to G
Almost as important is the hydrogen bonding in water and ice. No other chemical has such a peculiar liquid phase as H2O but then no other molecule is so ideally suited to forming hydrogen bonds. As each oxygen atom has two lone-pairs of electrons and two O-H bonds then each molecule can form four hydrogen bonds – the maximum possible for a molecule of only three atoms. This makes water versatile: it can crystallise in as many as eight different ways depending upon the temperature and pressure. Ordinary ice crystallises in an hexagonal structure which has large cavities (Figure 1). When ice melts the open structure partly collapses and this produces a liquid that is denser than the solid phase, so that ice can float on water. Warming water from 0 to 4 °C causes it to shrink in volume. This puzzling behaviour we now know is due to the further collapse of the hydrogen-bonding framework, which exceeds the increase in volume due to the thermal expansion.
The chemistry of hydrogen fluoride, HF, shows some similarities to water. It too has a much higher melting temperature (-92 °C) and boiling temperature (+19 °C) than would be expected by comparison with the heavier molecule hydrogen chloride, HCl, which melts at -112 °C, and boils at -84 °C. In solid HF the molecules are arranged in zig-zag chains held together by hydrogen bonds, and these persist in the liquid phase. When dissolved in water HF behaves as a weak acid, unlike HCl, and it has been assumed that the molecule does not readily dissociate as other acids do, but remains as free HF molecules. Recently this view has been challenged by Paul Giguere of Laval University, Quebec. He has suggested that HF dissociates in the same way as the compounds HCl, HBr and HI, collectively known as the hydrogen halides. In detail, HF+H2O → H3O+ + F-, except the oxonium ion does not associate with another water molecule to form H5O2+, but instead forms a strong hydrogen bond with the fluoride ion, H2O+-H-F-.
The fluoride ion F- should, and does, make an ideal centre for hydrogen bonding. If potassium fluoride, KF, is dissolved in liquid HF, in which it is extremely soluble, it forms the bifluoride ion [F-H-F]- also with strong hydrogen bonds. Salts of this ion have been known for more than 150 years and its structure was determined in the early days of X-ray crystallography. The fluorine atoms are only 226 pm apart and the proton is centred between them – a fact that was surmised in the original determination of the structure, and then proved conclusively by neutron diffraction in the 1950s.
For many years the strong hydrogen bond in the bifluorides was thought to be unique, but today we know of scores of such hydrogen bonds between oxygen, fluorine and nitrogen atoms. These strong bonds are characterised by the closeness of the two linked atoms. Normally two oxygen atoms cannot approach closer than 300 pm unless they are joined by a covalent bond. A normal O-H⋅⋅O hydrogen bond is about 300 pm long from oxygen to oxygen, but when forming a strong hydrogen bond, they can get as close as 240 pm or even less. The shortest such O-H-O bond has been measured at 229 pm in the hydrated oxide ion [HO-H⋅OH]-. This ion was first reported in 1979 by Kenneth Raymond of the University of California who discovered it in a chromium-complex salt. The oxonium ion too can form a strong hydrogen bond to a water molecule, [H2O-H-OH2]+. There are several crystals that contain this cation, and in most cases the hydrogen bond is about 244 pm long As for the fluoride ion F-, this is particularly adept at forming strong hydrogen bonds, not only to HF but to O-H and N-H as well.
Strong hydrogen bonds have emerged as a force to be reckoned with, challenging the very foundations of chemical theories of bonding. How can a hydrogen atom form two covalent bonds as it does in [F-H-F]-? It is theoretically impossible for a hydrogen atom to surround itself with two pairs of electrons and yet in this situation it clearly does so. Nor are these bonds weaker than other covalent bonds. In the bifluoride ion each hydrogen bond has an energy of approximately 400 kJ/mole, which is stronger than many other covalent bonds. Moreover, the hydrogen atom is not as fixed in position in a strong hydrogen bond as atoms in molecules generally are. The hydrogen need not be exactly centred, as it is in the bifluoride ion, but it can reside near one of the heavier atoms.
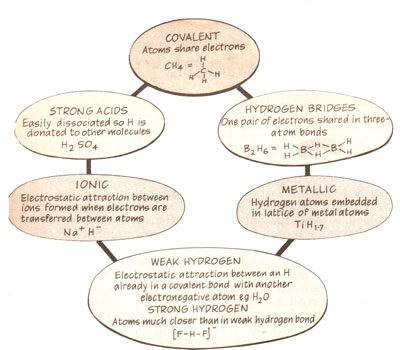 The chemistry of hydrogen
The chemistry of hydrogen- Hydrogen forms bonds with other atoms in six basic ways, although recent research has shown there to be two types of "hydrogen bonding" – weak and strong
How are strong hydrogen bonds detected? In addition to X-ray crystallography the techniques of infrared and nuclear magnetic resonance (NMR) spectroscopy can reveal their presence. The spectrum obtained when infrared radiation shines through a sample in which there are such bonds shows intense absorption – that is, whole regions of the spectrum may be blacked out because the strong hydrogen bond can interact with a wide range of electromagnetic radiation of infrared frequency, and not just a narrow range of frequencies as normal covalent bonds do.
NMR spectroscopy can be used to probe atoms in which the nucleus has a net intrinsic angular momentum, or spin, as does the single proton, and from NMR we can obtain information about the electron density surrounding the proton. Generally protons involved in hydrogen bonds are in "exposed" positions, in regions of low electron density, even in weak hydrogen bonds. In strong hydrogen bonds the NMR signals show the protons to be in the most exposed condition of any known compounds.
Strong hydrogen bonds are coming to light in organic and inorganic compounds, in solutions. in salts and even in natural minerals. It seems highly likely that they will have a role to play in biochemical systems. and recent discoveries are already pointing in that direction. Strong hydrogen bonds have been discovered at King's College, London, between the fluoride ion and naturally occurring organic acids such as those that partake in the Krebs cycle (the chemical reactions basic to aerobic respiration).
When potassium fluoride is added to a solution in water of succinic acid – one of the acids in the Krebs cycle – it will form crystals with a 1:1 ratio of KF and succinic acid. X-ray investigations of these crystals show a remarkable arrangement of alternating fluoride and succinic acid units, linked through strong hydrogen bonds 244 pm long. That such crystals grow from an aqueous solution suggests that these bonds would also form between these two species in vivo.
The amide group, CONH2 – another biologically important group – is also able to form strong hydrogen bonds with fluoride ions. James Clark of York University recently announced a uracil-fluoride strong hydrogen bond of this type. (Uracil is one of the compounds contained in ribonucleic acid (RNA).)
However, the fluoride ion shows only normal hydrogen bonding towards water molecules themselves. Fluoride salts dissolved in water show only weak chemical activity, and some of their insoluble salts have desirable properties. Thus the enamel of teeth, which is a variant of calcium phosphate known as hydroxyapatite, Ca5(PO4)3OH, is converted to fluorapatite Ca5(PO4)3F by treatment with fluoride. This salt is even less soluble than the original enamel and resists attack by dental caries more effectively.
On the other hand, too much fluoride in a diet is bad and can cause a condition known as fluorosis, which results in brittle bones. The fluoride collects in the skeleton because this too is chiefly calcium phosphate. Industrial exposure to high levels of fluoride in aluminium smelters and fertiliser plants has caused this condition in workers. Animals grazing in fields surrounding such plants and around brickworks, which also emit fluoride-containing smoke, have also been affected by fluorosis.
Low intake of fluoride is generally thought to be harmless, and indeed beneficial for dental hygiene, and claims that the low levels of 1 part per million recommended for public water supplies could cause allergy, cancer, birth defects and so on, have yet to be scientifically substantiated. Moreover the belief that fluoride is chemically inert when dissolved in water seemed a strong counter to such claims. But with the discovery that fluoride can form strong hydrogen bonds in the presence of water, we cannot be quite so complacent about its effect in the living cell, where a network of weak hydrogen bonds plays an important role in determining the structure.
The effects of fluoride on enzymes – nature's catalysts, which are responsible for controlling the body's chemistry – have been studied for more than 50 years. Fluoride stimulates some enzymes, but more usually, even in very low concentrations, it reduces their activity. Until recently the fluoride was thought to react with the metal atoms that are important components of many enzymes – the ability of fluoride to bond to metals is a well-known phenomenon. But recently researchers have discovered that fluoride can affect metal-free enzymes. Sonia Anderson of Oregon University has demonstrated the effect of fluoride on a metal-free enzyme and suggested that hydrogen bonding was the key to this behaviour. She also showed that another biologically-important compound, nicotinamide adenine dinucleotide, NAD – which is extremely important in channelling energy into living systems – was capable of binding to fluoride, presumably via a hydrogen bond.
Fluoride is clearly harmless to the growing number of people who take it in small doses from their drinking water – even bottle-fed babies appear to suffer no ill effects and they would be particularly vulnerable. But a warning bell has sounded: through the agency of the strong hydrogen bond fluoride can change the chemistry of many compounds. What it may be capable of doing in the living cell whether for good or ill remains to be discovered.